Make faster, more informed treatment decisions.

Every patient deserves the best treatment.
More than 200 targeted therapies are now approved for qualifying cancer patients.1 But fewer than 50% of lung cancer patients receive complete biomarker testing based on the current guidelines for non-small cell lung cancer (NSCLC).2
Existing tests take weeks to deliver results. Many samples fail, requiring repeat biopsies and additional delays. And the process can cost thousands of dollars.
Aspyre Clinical Test for Lung helps you make it possible.
The first application of the Aspyre technology, Aspyre Clinical Test for Lung enables fast, actionable treatment decisions for NSCLC patients.
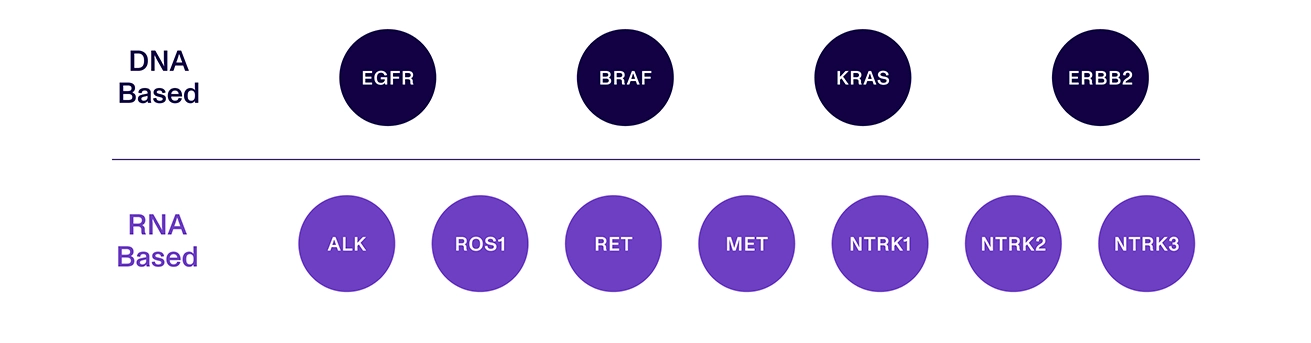
Aspyre Clinical Test for Lung tests all guideline-recommended genes associated with first-line targeted therapies for NSCLC.

Aspyre Clinical Test for Lung enables fast, confident treatment decisions.
Aspyre Clinical Test for Lung simultaneously analyzes DNA and RNA from blood or tissue in a single assay, maximizing the opportunity to identify DNA mutations and RNA fusions, while avoiding the additional time and expense of running separate assays.
Coverage of 114 variants across 11 genes, including DNA and RNA biomarkers