Globally decentralized biomarker testing.

Local, cost-effective biomarker testing in any lab.
The first research use only (RUO) application of our groundbreaking Aspyre technology, Aspyre Lung Reagents are designed to run on the existing global network of qPCR platforms, and can be seamlessly integrated into existing lab workflows anywhere in the world at a fraction of the cost of NGS.
Simultaneously analyze DNA and RNA from tissue or blood.
Easy to adopt and even easier to interpret, with turnkey data analysis, Aspyre Lung Reagents simultaneously analyze DNA and RNA from tissue or blood, maximizing the opportunity to identify mutations and fusions, while avoiding the additional time and expense of running separate assays.

Integrate seamlessly into your existing workflow.
The key to decentralized genomic testing, Aspyre Lung Reagents are designed to be integrated into your lab’s workflow with minimal staffing and instrumentation requirements.
Fast, simple, reliable biomarker testing on samples you control.
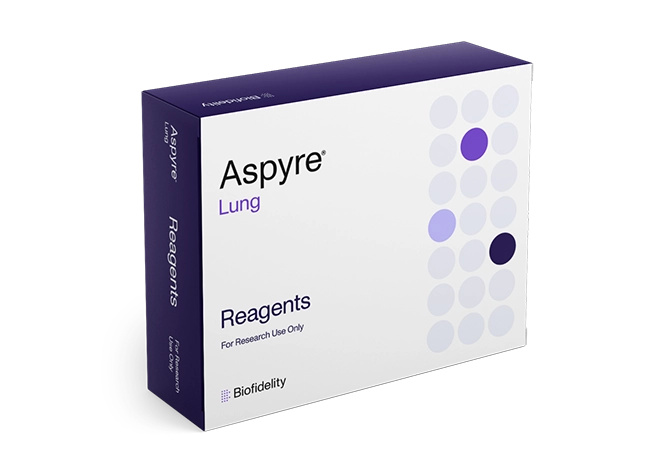
Using a simple, 4-step workflow on qPCR platforms, Aspyre Lung Reagents can deliver results in hours from 114 variants across 11 genes, including DNA and RNA biomarkers, to aid in NSCLC research.
Coverage of 114 variants across 11 genes, including DNA and RNA biomarkers
Simple setup & consistent performance across sites, including challenging sample types
Multi-site Concordance
- Fast setup at University of Pennsylvania and Medical College of Wisconsin
- 54 FFPE tissue samples analyzed at University of Pennsylvania, Medical College of Wisconsin and Biofidelity
- Excellent concordance between sites
Challenging Sample Types
- 16 additional ‘challenging’ cytology samples tested at all sites
- Included peritoneal fluid, pleural effusions, fine needle aspirates (FNAs) & FNA washes
Aspyre Lung Reagents by the numbers
DNA
SNVs & Indels
RNA
Fusions
MET
Exon 14 skipping
Sensitivity
Median panel-wide LoD95
1.10% VAF
8 amplifiable copies
262 amplifiable copies
Specificity
100%
100%
100%
DNA
SNVs & Indels
RNA
Fusions
MET
Exon 14 skipping
Sensitivity
Median panel-wide LoD95
0.19% VAF
1 amplifiable copy
69 amplifiable copies
Specificity
100%
100%
100%
Aspyre Clinical Test for Lung
The first clinical application of the Aspyre technology, Aspyre Clinical Test for Lung provides fast, actionable biomarker data for non-small cell lung cancer (NSCLC) patients.